

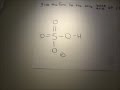
















In this example, sulfuric acid (H 2SO4) is an acid because it "donates" H + to the water. It becomes the hydrogen sulfite ion (H SO− 4) which is the conjugate base of sulfuric acid. The same idea applies to a base: N H 3 + H 2O <=> N H + 4 + OH − Here's what I got. The thing to remember about conjugate acids is that they are the chemical species that is formed when a Bronsted-Lowry base accepts one proton, "H"^(+). This means that in order to find the conjugate acid of a substance that can act as a Bronsted-Lowry base, all you have to do is add a proton to it. But keep in mind that a proton carries a 1+ charge, so make sure that you ... In each of the following chemical equations, identify the conjugate acid-base pairs. a. HSO4- + H2O ( SO42- + H3O+ b. CH3NH2 + HCl ( CH3NH3+ + Cl-c. CH3COO- + H2O ( CH3COOH + OH-Complete the equation for each of the following conjugate acid-conjugate base reactions. HNO2 + H2O ( H3O+ + NO2- Which is a conjugate acid-base pair in the following equation? H2SO4 + H2O ----> HSO4 -1 + H3O +1 a H2SO4 and H2O b H3O +1 and H2SO4 c HSO4 -1 and H3O +1 d H2SO4 and HSO4 -1 what is the conjugate acid for ClO4^-what is the conjugate base for H2SO4. what is the conjugate acid for HSO4^- Click here👆to get an answer to your question ️ The conjugate base of HSO4^- in an aqueous solution is: Problem: Identify the conjugate acid of SO42–.a. H2SO4b. HSO4–c. H2SO3d. H3O+e. SO32– FREE Expert Solution Show answer. 80% (240 ratings) Problem Details. Identify the conjugate acid of SO 4 2–. a. H 2 SO 4 b. HSO 4 – c. H 2 SO 3 d. H 3 O + e. SO 3 2– Learn this topic by watching Conjugate Acids and Bases Concept Videos. All Chemistry Practice Problems Conjugate Acids and Bases ... While in theory HSO4- the conjugate base of sulfuric acid, in aqueous solution it will never actually be a base, because it won't accept a proton to make molecules of H2SO4. As I explained, H2SO4... What is the conjugate base of hso4? HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. The conjugate base of HSO4- is SO4 (2-), if that’s the question that you are asking. Is CN an acid or base? What is the conjugate base of HSO4−? Express your answer as a chemical formula. What is the conjugate acid of HPO42− ? Express your answer as a chemical formula. Videos. Step-by-step answer 100% (2 rating) 01:43 2 0. Expert Answer 98% (66 ratings) Previous question Next question ...
[index] [3143] [1802] [9232] [383] [5178] [2925] [2122] [9571] [5250] [2101]
Acid Base Chemistry 4c. ... or 4 electronegative oxygen atoms present, as in the case of the HSO4- ion, ... 7a. pKa and pKb of conjugate acids and bases - Duration: 6:46. Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com Draw CH3NO2 (Nitromethane) in Lewis and its conjugate acid - Duration: 61 seconds. 11,751 views; 4 years ago; 1:04. Give the formula of the conjugate acid/base of HSO4^(-) - Duration: 64 seconds ... Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks Covers the HSC chemistry syllabus dot point: "identify amphiprotic substances and construct equations to describe their behaviour in acidic and basic solutions" About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... Identify the conjugate acid-conjugate base pairs in the following chemical equations:HCOOH (aq) + CN- (aq) = HCOO- (aq) + HCN (aq)H2SO4 (aq) + N2H5+ (aq) = H... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 068 - Acid-Base EquilibriumIn this video Paul Andersen explains how acid-base chemistry can be understood in terms of equilibrium. Water is present in all a...
Copyright © 2024 top100.onlinerealmoneygames.xyz